
Estimating Seed Yield and
Duck-energy Days in Moist-soil Wetlands

Background
Waterfowl biologists estimate seed
production in moist-soil wetlands located along migration routes and at
wintering sites to calculate duck-energy days (DEDs). Duck-energy days are the number of dabbling
ducks (tribe: Anatini)
that potentially can be sustained energetically in a wetland for a specified
duration. Waterfowl biologists also
estimate seed production in moist-soil wetlands to monitor plant succession and
to evaluate management techniques. Thus,
obtaining accurate estimates of seed production in moist-soil wetlands is
critical in calculating DEDs, monitoring plant succession, and evaluating
waterfowl management.
Seed
production can be estimated directly by harvesting plants in plots located
across a moist-soil wetland, threshing seeds from plants, and drying and
weighing threshed seeds. However, direct
estimation of seed yield is very time consuming and requires a drying oven and
balance. In the 1990s, scientists
developed equations that used plant measurements (e.g., plant height, seed head
diameter) to estimate seed production of moist-soil plants (Laubhan 1992,
Laubhan and Fredrickson 1992, Gray et al. 1999a, Sherfy and Kirkpatrick 1999).
However, waterfowl biologists were reluctant to use these models because
measuring multiple plant parts was tedious and time consuming. Gray et al. (1999b) proposed a new method for predicting seed production of
moist-soil plants using one simple variable: the number of dots on a grid
covered by seed on a seed head. Models
developed using the dot-grid method predicted seed production accurately within
and outside the Southeast region (Gray et al. 1999a, Anderson 2006). However,
similar to previous studies, few biologists used dot-grid equations because
counting dots was tedious and time consuming.
Counting number of dots on a grid
covered by seed is an index of seed-head area.
Portable and desktop
scanners are used frequently by the forestry industry to estimate leaf area and
can be used to quantify area of a seed head.
Thus, Gray et al. (2009) used this technology to develop new equations
that predicted seed production per plant using scanned seed-head area for both
scanner types. They also compared
predictive ability of the equations and time spent processing samples between
scanner types and the dot-grid method proposed by Gray et al. (1999b).
All equations explained substantial variation in seed mass (R2 ≥ 0.87) and had high predictive ability. However, processing time of seed heads
averaged 22 and 3 times longer for the dot grid and portable scanner,
respectively, than for the desktop scanner.
Processing time was longest for the dot-grid method, averaging >5
minutes per plant, with some species requiring >10 minutes. In contrast, processing time averaged 45
seconds and 15 seconds per plant for portable and desktop scanners,
respectively. Thus, Gray et al. (2009)
recommended use of desktop scanners for accurate and rapid estimation of seed
production in moist-soil wetlands.
Inasmuch as the desktop scanner used in this study (LI-COR
LI-3100) costs $9,600 (in 2009 USD), they suggested that dot-grid equations
could be used if funds were unavailable to purchase a scanner (Gray et al.
2009). Seed predictions per plant from
the equations in Gray et al. (2009) can be multiplied by mean plant density to
estimate total seed production and DEDs in moist-soil wetlands. These procedures are outlined below and an
Excel file provided to facilitate easy calculation.
Estimating Aboveground Seed Production and
DEDs
STEPS:
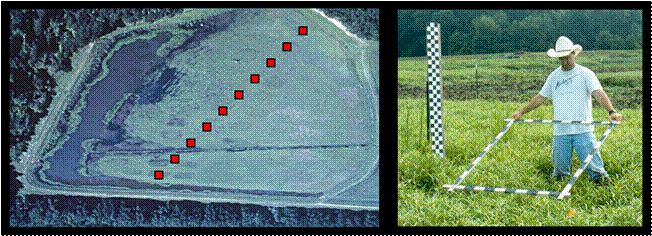
1.
Establish a minimum of ten 1-m2
plots across a moist-soil wetland.
The easiest design is systematically placed plots along a transect (left
image below), although plots could be placed randomly or following a different
statistically sound sampling design (e.g., stratified random). Sampling should occur when the majority of
plants have produced seed heads but prior to seed dislodging from heads (i.e.,
typically in September or October depending on latitude).
2.
Count the number of seed-producing plants
separately for each species. Only count
plants that produce seed for dabbling ducks.
The goal of this step is to estimate plant density across the wetland
for each species.
3.
Randomly select one plant per species
(counted in #2), clip all seed heads from it, and place seed heads in separate
plastic bags that are labeled with the plot number and plant species. NOTE: If a plant contains >1 seed
head, clip and collect all seed heads because the objective will be to estimate
seed yield per plant. Also, random
collection of plants is encouraged so not to introduce observer bias (e.g., picking
plants with larger seed heads). One approach
is dividing the 1-m2 plot into a numbered 10-decimeter grid and
collecting the nearest plant to a randomly generated intersection of two
numbered decimeters (see right image below, yellow arrow showing a random
intersection).

4.
After all plots are sampled and you
returned from the field, seed heads should be placed in a plant press and
stored at room temperature for one or more weeks. This step is not necessary although
scans are more consistent and it is easier to count dots if seed heads are
pressed.
5.
Scanning:
For each species, scan the seed head(s) from the randomly selected plant at
each plot. If the plant
contained >1 seed head, sum the scanned area (cm2) across heads. The default scanning units for both scanners
is cm2 so no post-scanning conversion is necessary if default units
are used. If the ADC AM300 portable
scanner is used, set the contrast on 5 for all species, except rice cutgrass (Leersia oryzoides), which should be
scanned at contrast 3. Prior to
scanning, remove all leaves and trim the plant stem so it is approximately
flush with the bottom of the seed head.
Other plant parts (e.g., side stems [pedicels] that contain seed) do not
have to be removed. If the seed head is
too large to fit on the scanner, cut it into sections, scan each section, and
sum the area across sections. NOTE: Multiple
scans may be necessary with the ADC AM300 to produce a clear scanning image
(seen output window in center image below).
Multiple scans with the LI-COR LI-3100 desktop scanner (right image) are
unnecessary, which increases the rate that samples can be processed with this
scanner (i.e., approximately 15 seconds per plant).

Dot Counting:
Procedures are identical to scanning except count the number of dots that are
covered by seed for each randomly collected seed head per plant species per
plot. If the plant contained >1 seed head,
sum the number of dots across
heads. Only dots that intersect seed
(not stems or pedicels) and cover over 50% of the dot area should be
counted. The dot grid (left image above)
contains 9 dots/cm2 and can be created by typing periods using
bolded Courier font (20 pt) with 0.5 line spacing. Free grids can be requested from M. Gray (mgray11@utk.edu). For easier counting, a transparency can be
made of the grid and overlaid on the seed head.
6.
Average the scanned seed-head area (or
number of dots) across the plots for each plant species. Average only the plots that contained
seed heads for a particular species. The goal of this step is to
incorporate the natural variation in seed production across the wetland for
each plant species into equation predictions.
7.
Average the number of plants counted for
each species across sampling plots. If a plot
did not contain a particular species, its density = zero thus zero should be
included in the average for that plot. The goal of this step is to
incorporate the natural variation in plant density for each species across the
wetland into equation predictions.
8.
Excel File for Seed
Production and DED Predictions: (right click on link and select save
to download)
Data Needed: 1)
Information from #6 and #7 above.
2) Acreage (in hectares) of moist-soil wetland.
Spreadsheet
Structure:
1)
There are 3 columns
(colored blue) to enter the above data.
2)
For each plant
species, there are 3 rows each corresponding to a seed prediction method (dot,
portable scanner, or desktop scanner).
9.
Enter averages and acreage into the
appropriate row and column; leave all other cells blank. For each plant
species, there should be one row of data entered (corresponding to the method
used). If a plant species in the
spreadsheet was not found, do not enter any data. If you collected a seed-producing plant
species that is not included in the spreadsheet, a plant species with a similar
seed-head shape could be used (e.g., common barnyard grass for Japanese
millet).
10.
Calculations
There are 4 predictions made in the
Excel spreadsheet:
1)
Kg of seed (dry mass) produced per hectare
Calculated using prediction
equations in Table 1 in Gray et al.
(2009). Seed mass (g) predictions
per plant from the equation is multiplied by plant density and converted to kg
seed per hectare by multiplying by 10 (i.e., simultaneously converts g to kg
and m2 to ha).
2)
DED per hectare
Calculated using the equation
below. Seed production is estimated in
#1 using prediction equations and plant density. The true metabolizable energy (TME) of seed
for each plant species is from Kaminski
et al. (2003). If a value was not
available for a plant species in this publication, 2470 kcal/kg was used, which
is the standard TME used for moist-soil seed (Reinecke et al. 1989). Daily energy requirement for a mallard (292
kcal/day) was used, which is standard (Reinecke et al. 1989).

NOTE: Commonly, 50 kg/ha is subtracted from
the above DED estimate to account for the “giving-up” density of food
resources, which is when waterfowl abandon foraging sites because it is no
longer energetically profitable (Greer et al. 2009). The Excel file does not perform this
calculation; however, users can account for this threshold if desired by
subtracting 50 kg/ha from the total seed produced/ha or by subtracting 423
DED/ha from the total DED/ha (i.e., summation values in the YELLOW cells of the
Excel spreadsheet).
3)
Total kg of seed produced
Estimates in #1 are multiplied by
acreage (ha) of the moist-soil wetland.
NOTE: Calculations should be
performed separately for each moist-soil wetland on an area to account for
spatial variation in seed production.
4)
Total DED
Estimates in #2 are multiplied by acreage
(ha) of the moist-soil wetlands.
NOTE:
To incorporate the giving-up threshold for the entire wetland, multiply 50
kg/ha or 423 DED/ha by total wetland acreage (ha) and subtract this value from
the corresponding totals given in the Excel sheet (see TAN cells).
PowerPoint Presentation: New Technology
to Estimate Seed Yield (see Slide 23 for fees)
Assistance with
Calculations
Matthew J. Gray, Ph.D.
Email: mgray11@utk.edu
Phone: (865) 974-2740
Processing Service Available: Fast and Easy!!

Example of Seed
Production and DED Estimates Report Produced by UT Wetlands Program.
References
Anderson, J. T. 2006. Evaluating competing models for predicting seed mass of Walter’s millet. Wildlife Society Bulletin 34:156-158.
Gray, M. J., R. M. Kaminski, and G. Weerakkody. 1999a. Predicting seed yield of moist-soil plants. Journal of Wildlife Management 63:1261-1268.
Gray, M. J., R. M. Kaminski, and M. G. Brasher. 1999b. A new method to predict seed yield of moist-soil plants. Journal of Wildlife Management 63:1269-1272.
Gray, M. J., M. A. Foster, and L. A. Peña Peniche. 2009. New technology for estimating seed production of moist-soil plants. Journal of Wildlife Management 73:1229-1232.
Greer, D. M., B. D. Dugger, K. J. Reinecke, and M. J. Petrie. 2009. Depletion of rice as food of waterfowl wintering in the Mississippi Alluvial Valley. Journal of Wildlife Management 73:1125-1133.
Kaminski, R. M., J. B. Davis, H. W. Essig, P. D. Gerard, and K. J. Reinecke. 2003. True metabolizable energy for wood ducks from acorns compared to other waterfowl foods. Journal of Wildlife Management 67:542-550.
Laubhan, M.
K. 1992.
A technique for estimating seed production of common moist-soil plants.
Laubhan, M. K., and L. H. Fredrickson. 1992. Estimating seed production of common plants in seasonally flooded wetlands. Journal of Wildlife Management 56:329-337.
Reinecke, K. J.,
R. M. Kaminski, D. J. Moorhead, J. D. Hodges, and J. R. Nassar. 1989.
Mississippi Alluvial Valley.
Pages 203-207 in L. M. Smith,
R. L. Pederson, and R. M. Kaminski, editors.
Habitat management for migrating and wintering waterfowl in
Sherfy, M. H., and R. L. Kirkpatrick. 1999. Additional regression equations for predicting seed yield of moist-soil plants. Wetlands 19:709-714.
UT Department of
Forestry, Wildlife and Fisheries

